|
Batteries are assembled
from cells, connected in
series, to increase the
voltage available.
In a cell chemical
energy is converted into
electrical energy. Cells may be either
PRIMARY or SECONDARY
types.
A primary cell is
discarded when its
chemical energy is
exhausted.
A secondary cell can be
recharged.
The most common primary
cell is the zinc/carbon
(Leclanche) as used in
torches, portable radios
etc.
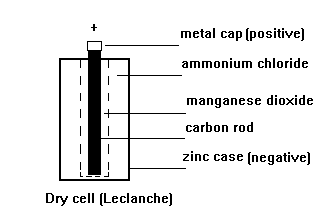
The zinc and carbon
react with the ammonium
chloride ELECTROLYTE to
produce electricity.
The manganese dioxide
absorbs hydrogen gas
produced around the
carbon rod which would
insulate it from the
electrolyte and stop the
cell working.
The most common
secondary cells are the
lead/acid and
nickel/cadmium (nicad).
Lead acid batteries need
a constant voltage
charger.
Nicads must be charged
with a constant current
charger.
All cells have INTERNAL
RESISTANCE.
This is not an actual
resistor but a
characteristic of the
cell.
Internal resistance
increases as the cell
ages.
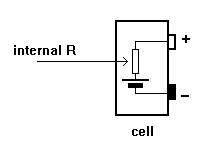
When current is taken
from a battery, voltage
is dropped across this
internal resistance and
the voltage at the
battery terminals falls.
The diagram shows that
as the current taken
increases the terminal
voltage decreases.
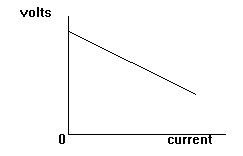
This is called POOR
REGULATION.
It occurs in any type of
power supply.
Battery voltages must
therefore always be
measured ON LOAD, i.e.
with the radio etc
switched on and drawing
current. |